Inge Pascoe & her children – on the way to expansion
Kapitel IV: 1970-1999
32. New leadership and reorganisation
After the death of Fritz Pascoe in June 1970, his wife Inge suddenly found herself at the helm of the Gießen pharmaceutical company Pascoe GmbH at the age of 52 years. “Sale was out of the question for me,” the senior boss recalls. “I decided to keep the company in the family, to pass on later to our then 14-year-old son.” And she secured support: in 1971, Inge Pascoe’s daughter from her first marriage, Dr. Birgit Wilrich, entered the company. The graduated pharmacist had been working until then as a research associate at Eberhard Karls University Tübingen. “It went without saying that I would help my mother”, Birgit Wilrich recounts, who was responsible for the manufacturing and quality control departments for over 30 years.
With the experienced procurator Fritz Arnold by her side, Inge Pascoe took over the pharmaceutical company at a time when homeopathy was gaining recognition worldwide. Doctors and patients were increasingly questioning the effectiveness, manufacturing quality and side effects of medicines. It was soon clear to the new management that they had to reorganise production in the face of increasing quality and market demands. In 1969, the World Health Organisation (WHO) had first published the GMP rules (Good Manufacturing Practice): with requirements for hygiene, premises, equipment, documentation and controls in the manufacturing of medicines. Although Pascoe had already set up a research division at the beginning of the 1960s, which documented and provided reproducible data for scientific proofs of effectiveness, the goal now was to align the entire production procedure, from the manufacturing of substances to the multiple medicine quality controls, with the GMP guidelines.
Birgit Wilrich contributed the necessary know-how for this. In close consultation with her mother, she reorganised work processes and had production spaces restructured. An air pressure procedure with a special ventilation system was installed, which guaranteed absolutely germ-free air in the production machine area. Birgit Wilrich drove the rationalisation of work processes forward with the installation of a fully automated filling and packing channel. The new system could fill up to 3600 bottles in an hour at medium speed. There were special air pressure conditions under a suspended foil that ensured that this area remained sterile. Birgit Wilrich also extended quality controls in the chemical-analytical control laboratory in accordance with the latest scientific findings. Together with a qualified food chemist, she addressed the preservability of the remedies and the standardisation of the basic materials. To do so, Pascoe invested in the latest equipment such as polarimeters, pH measuring devices, leak tests and systems for evaluating chromatography and capillary analytical procedures.
The reorganisation of production was successful. When Pascoe was audited in 1974 according to the WHO guidelines for all pharmaceutical industrial enterprises, there were “no objections”. In the same year, the company with ist 85 employees was able to achieve an annual turnover of around 6 million Deutschmarks.







33. Proven and new products
In the mid-1970s, Pascoe’s production programme comprised infusions, specialities and injections. To manufacture the plant extracts, plants that were either dried or delivered immediately after harvesting were milled and processed with alcohol. The laboratory staff examined the extracted material to set the tinctures according to the specifications in medical books and company regulations. A special hydraulic press produced fresh plant juices and tincture bases. The fresh plants were now not always sourced from the region. Since the expansion of the production buildings in the 1960s, there was no fresh plant garden anymore on the company premises at Schiffenberger Weg. Birgit Wilrich wanted to change this: “Taking up fresh plant cultivation and manufacturing the tinctures ourselves again was very important to me for quality reasons.” She looked for a suitable agricultural enterprise nearby that fulfilled Pascoe’s high quality requirements. She found it at the herb farm Kräuterhof Müller in Staufenberg, just six kilometres from the Gießen manufacturing plant.
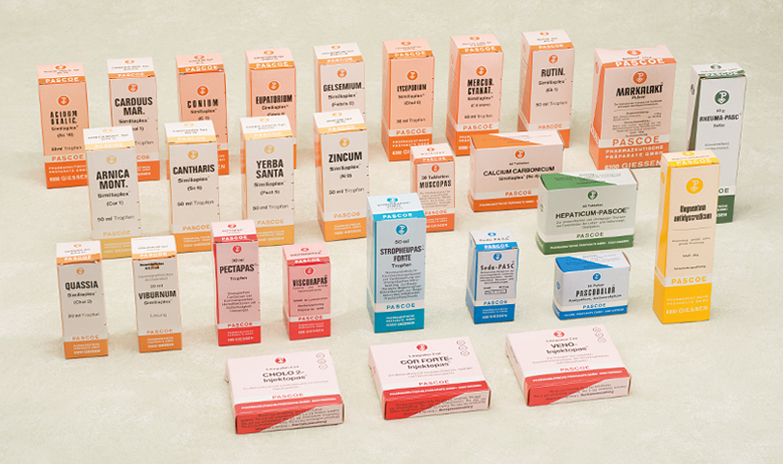
Pascoe continuously extended its portfolio of plant-based and homeopathic remedies also in the 1970s. Dr. Helmut W. Schimmel tested the use of complex nosodes in medical practice. Nosode therapy is a very specific form of homeopathic stimulation therapy. It is used in the areas of early diagnosis and early treatment as well as for chronic diseases. Dr. Helmut W. Schimmel defines nosodes as “sterilised pharmaceuticals potentised according to homeopathic rules”. The starting materials are tissue, secretions, excretions, serums and blood elements, devitalised microbial cultures of viruses, bacteria and fungi, as well as parasites. In close cooperation with the research division, he developed the so-called noso complexes, which traded as from 1975. These complexes contained the aforementioned tissue and microbe nosodes as well as mixes of homeopathically prepared, commonly occurring toxic substances such as heavy metals, insecticides or pesticides. Pascoe was thereby responding to the possible effects of the increasing environmental pollution.
«Taking up fresh plant cultivation and manufacturing the tinctures ourselves again was very important to me for quality reasons.»Birgit Wilrich
34. Goodness is so nearby – raw materials from Staufenberg
Pascoe attaches great importance to environmentally friendly production. This starts with the choice of raw materials for the plant-based and homeopathic remedies. The Gießen pharmaceutical manufacturer sources the majority of local healing plants from the nearby herb farm Kräuterhof Müller in Staufenberg.
The successful cooperation started in the 1970s. Since 1987, agricultural engineers have been cultivating medical plants such as arnica, amber, daisies, milk thistle, calendula, echinacea, tiger lilies and knotty figwort according to controlled organic guidelines. The planting of high-quality raw materials in the region, the harvesting by hand, the short transport routes and the immediate processing at Pascoe are guarantees for consistent quality and product safety.



35. “Without male support”
To win new customers, Inge Pascoe and her daughter also modernised the marketing strategy of the company. Pascoe took part with information stands in the annual congress of the Central Association of Doctors for Natural Medicine and Regulation Medicine (ZAEN) in Freudenstadt, as well as the German Cancer Congress in Bad Salzuflen. Pascoe also visited many national health practitioner congresses.
Dr. Helmut W. Schimmel and Prof. Dr. Horst F. Herget, who had since been awarded a professorship for anaesthesia at Justus-Liebig University Gießen, presented their results here for iridodiagnosis, phytotherapy and chronic complaints, as well as pain disorders, which they had worked on together with Pascoe. In monographs partly comprising several volumes, the two scientists summarized the results for the interested general public. The books, which often achieved many print runs, were published by the scientific division of Pascoe Pharmazeutische Präparate GmbH.
When the managing director Friedrich Arnold no longer wanted to go along with Inge Pascoe’s modernisation drive, she parted ways with her longstanding colleague, a brave decision at the time. In the 1970s, it was considerably rarer than today for women to be heading up a company. “There was simply a lack of confidence in me and my daughter being able to run the company without male support,” Inge Pascoe summarises the mood at the time. From now on, Birgit Wilrich was also responsible for purchasing alongside production. Inge Pascoe took over finances. She had acquired the necessary qualification since the death of her husband through evening courses in business administration. “Accounting was especially important to me, because I wanted to bring it up to speed,” Inge Pascoe recounts.
«There was simply a lack of confidence in me and my daughter being able to run the company without male support.» Inge Pascoe
36. The second pharmaceutical law – a revolution
When the social-liberal coalition under Helmut Schmidt announced the law for the restructuring of pharmaceutical law in September 1976, due to increased international requirements for the safety of pharmaceuticals, the two women were facing their first big challenge.
“The passing of this law was quite a revolution for the pharmaceutical sector,” Dr. Birgit Wilrich recalls. Hitherto it had been comparatively easy to introduce a new pharmaceutical. The Pharmaceutical Law (AMG) of 1961 did not include an obligation to test pharmaceuticals for effectiveness and tolerability; there was only registration. “For industrial pharmaceutical production, which then produced 85 percent of all medicines, nothing was regulated at all,” she describes the situation.
The Second Pharmaceutical Law, which came into force on 1st January 1978, set out the principles for quality control for the first time, as well as for the pharmacological-toxicological and clinical testing of remedies. To increase the safety of pharmaceuticals, manufacturers now had to go through an approval procedure. The new guidelines were quality, effectiveness and safety.
For Pascoe, documentation of the manufacturing processes and analytical controls of the remedies had for a long time been par for the course. Even so, the new law represented a turning point for the Gießen company. “For us as a company specialising in natural medicine, the new law was important because it set out that plant-based and homeopathic pharmaceuticals could continue to be prescribed,” Dr. Birgit Wilrich recalls. It classified natural medicines as a special therapy orientation.
This meant that the Second Pharmaceutical Law, contrary to the initial proposals that would have meant either an elimination of or discrimination against homeopathic and phytotherapeutic pharmaceuticals, brought registration for homeopathic remedies and facilitated approval conditions for phytotherapeutic remedies. As plant-based pharmaceuticals are mixed of various substances, however, proof of effectiveness through pharmacological studies for phytopharmaceuticals was not possible. The Federal Ministry of Health therefore recognised usage observations, medical experience reports or collections of individual case reports for natural medicine products. The products were given registration numbers and the manufacturers were allowed to attribute clear indications to them, which were stated on the packaging.
As part of the reform of pharmaceutical law, the first official Homeopathic Pharmacopoeia (HAB 1) was also published in 1978. For this purpose, the Federal Ministry for Youth, Family and Health had convened the Homeopathic Pharmacopoeia Commission with two committees. Dr. Birgit Wilrich was a member of the analytics committee. “The main objective was quality improvement,” is how she describes the task of the commission. “We set out procedures and study solutions with which medical raw materials could be checked for their identity and purity and drying losses could be determined.” The identity and purity tests were intended to exclude impermissible additions of other types of plants or contamination through non-plant additions. HAB 1 bindingly regulated the manufacturing of all pharmaceuticals made according to homeopathic procedures.
37. Changed product palette
The new law brought not only clarity when it came to quality standards but also a huge cost for meeting them. The retrospective authorisation of already introduced medicines dragged on for more than two decades, for some Pascoe products until recent times. The procedures took so long because at the beginning of the 1980s, only around ten percent of all healing plants had been pharmacologically studied. The Federal Ministry for Youth, Family and Health convened a committee of experts for plant-based pharmaceuticals (Commission E) which gathered scientific and practice-based material on healing plants for so-called monographs, the basis for new and retrospective registrations permits. The monographs described the effective components of the pharmaceutical, its pharmacological properties, as well as injections, homeopathic single substance injections, nosode usages, side effects, dosage recommendations and types of use.
Despite the preliminary work by the in-house research division since the 1960s, the retrospective registration represented increased testing costs for Pascoe. For each pharmaceutical, detailed analytical examination results had to be presented, with far-reaching consequences for Pascoe’s product palette. “Many of our pharmaceuticals contained numerous ingredients. We then reduced them to one to three substances,” Birgit Wilrich recalls. “The individual product now contained higher proportions of the respective substances. This also led to a pharmaceutical becoming a nutritional supplement.” A further consequence of the Second Pharmaceutical Law for the product palette was a reduction in the number of products offered. Only around 250 of the 2500 pharmaceuticals remained. The reason: before the new law, Pascoe had put together numerous pharmaceuticals according to the individual requirements of various health practitioners, which was no longer possible after the introduction of the monographs.
At the beginning of the 1980s, Pascoe was offering products from six groups: specialities in various administration forms, homeopathic mixed injections, vitamin complexes and similiaplexes. The pharmaceuticals covered a wide spectrum of clinical symptoms: cardiovascular disorders, gastrointestinal complaints, nervous disorders or kidney and bladder diseases. The areas of use ranged from cough mixture to the rheumatism remedy Rheumapasc and products against acute complaints, chronic illnesses and those for strengthening endogenous immunity such as Pascotox.
38. Export orientation under junior boss Jürgen F. Pascoe
To remain competitive into the future, Pascoe had to raise productivity and rationalise further. The company invested in an EDP-assisted controlling system on an absorbed cost basis. Because wages and social contributions had increased greatly during the 1970s, profit had reduced. Inge Pascoe therefore felt forced to let go of employees. By 1983, the number of employees had declined to 67. At the same time, Pascoe modernized its production facilities according to GMP guidelines and invested in comprehensive building measures. As a lack of warehousing space limited production volumes, a new warehouse and dispatch building was constructed. Pascoe also enlarged the company premises.
The fact that the company new build could be inaugurated in 1984 after just a few months of building time was also thanks to the junior boss Jürgen F. Pascoe. After completing his studies, the qualified industrial engineer had entered the parental company in 1983. From 1987, he headed up the family enterprise together with his mother as managing director. Whilst the previous corporate strategy had been orientated towards raising quality and rationalising production, Jürgen F. Pascoe set his sights on export. Although Pascoe was already supplying individual pharmacies in the USA, Scandinavia and the Netherlands, the export rate stood at only 3.4 percent in 1982. Jürgen F. Pascoe saw great export opportunities for natural medicines made in Gießen, not least because there were more and more individual orders from foreign medics and enquiries from doctors in the Anglo-American region, seeking sales rights for Pascoe products.
Jürgen F. Pascoe drew up a step-by-step, differentiated export marketing concept and a detailed analysis of the US market. Because in the 1980s, American homeopathy was experiencing a renaissance. At the international doctors’ congress for alternative medicine in Baden-Baden, Jürgen F. Pascoe forged initial contacts with US doctors and a Californian sales company. At the same time, he made an increasing effort to find buyers in other English-speaking countries such as Canada, South Africa, Australia, New Zealand and India. Through Dr. Helmut W. Schimmel, he got to know the British medic George Lewith, who is today Professor of Health Research at the Department of Primary Care at the University of Southhampton. From 1980 to 2010, Lewith was co-owner of the Centre for Complementary and Integrated Medicine, as well as of a medicine wholesaler with which Pascoe cooperates to this day. Jürgen F. Pascoe also systematically developed sales in German-speaking neighbouring countries and made contact with pharmaceutical wholesalers in western Switzerland and in Linz in Austria. At a trade fair, he made acquaintance with an Italian entrepreneur who had personally experienced the healing effect of Pascoe remedies. A close business relationship soon developed with the sales company based to the north of Milan, which continues up until today. Jürgen F. Pascoe drove the new export strategy forwards with great commitment: in 1985 Pascoe was already delivering to more than 30 countries worldwide. In 1987 the company founded the subsidiary Planta-Pharm Arzneimittel Vertriebs GmbH.
«We wanted to avoid the use of pesticides and herbicides in the cultivation of healing plants for our pharmaceutical production.» Birgit Wilrich

39. “Is there not also a natural medicine instead?”
The prescription books of Pascoe in the English language met with great demand abroad. The presentations that an employee at the scientific division in England and in the USA held on the subject of natural medicine diagnosis and treatment methods were also received with wide interest. After his return, he reported to colleagues in Gießen that the reactions had been thoroughly “enthusiastical”.
And what was the situation like in the Federal Republic? Pascoe also registered a “strong positive trend in the population” on the German market. According to an Allensbach survey from the year 1989, natural medicines, in particular phytotherapeutic remedies, were in fashion and were evaluated positively by 58 percent of those questioned. While in 1980 plant-based products had only had a proportion of around 7.7 percent of the total turnover for all pharmaceuticals, in 1989 it was already 10 percent. The now nine Pascoe foreign sales representatives heard the question increasingly frequently: “Is there not also a natural medicine instead?” More and more patients wished to be prescribed natural medicines. At the same time, awareness was growing regarding the plant protection agents used in agriculture. “We wanted to avoid the use of pesticides and herbicides in the cultivation of healing plants for our pharmaceuticals production,” Birgit Wilrich recalls. “That is why in 1987 we decided on controlled organic cultivation and careful harvesting methods.”
With numerous publications, Pascoe informed their customers in what cases natural therapy methods could be successfully used. Not only among the population, but also among doctors and medical students, interest in natural medicine treatments was growing. In the 1980s, the number of members of the German Central Association of Homeopathic Doctors tripled. For medical practitioners there were new further training possibilities and for students up-to-date learning material on phytomedicine. In addition, various universities were offering more educational events on the subject of homeopathy. In 1988, homeopathy and natural medicine were included as examination subjects in the medical licence for doctors. The Open University established the first professorship for natural medicine in West-Berlin in October 1989.

40. Quality, research and development
In order to ensure the highest possible product quality in the long term, Pascoe invested even more in internal quality controls. To avoid mutual contamination of plant-based and homeopathic pharmaceuticals, the company started to manufacture injection solutions, tablets and ointments in different areas. The identity and purity of the products were monitored both during the manufacturing processes and with regard to the end products with the latest chromatographic and spectrometric procedures. Furthermore, Pascoe automated the manufacturing processes on account of the high hygiene requirements: ampoules for injection solutions were cleaned in a closed system, sterilised before and after filling and then subjected to computer-aided testing for any particles and the correct filling quantity. The balm preparation took place under vacuum to protect the oxidation-sensitive substances against air oxygen. The wrapping of the dragées and tablets, as well as the filling and sealing of ointments in tubes were now fully automated. In addition, Pascoe made use of specialist personnel for additional visual checks and built up a computer-aided commissioning alley for a high-efficiency and quick delivery service.
As a guarantee of consistent quality and production safety, Pascoe also invested increasingly in long-term clinical studies. Since the 1960s, the research division had carried out studies on the effectiveness and tolerability of natural medicines, in close cooperation with clinics, local doctors and natural health practitioners. In 1985, Pascoe presented the clinical study on the liver-gall bladder remedy Legapas, a plant-based and homeopathic combination product, drawn up in accordance with the guidelines of the German Association for Internal Medicine. “Legapas, whose effect is thanks to cascara rind, was then one of our bestselling remedies,” is how Birgit Wilrich explains the selection of the remedy. From 1965 to 1985, 99 testers examined around 1100 patients. They established the therapy effects on six indications, including gallstones, jaundice and liver infections.
Under the direction of Dr. Birgit Wilrich, Prof. Dr. Horst F. Herget, Dr. Helmut W. Schimmel and a Pascoe employee evaluated the data after the treatment with Legapas. The complaints disappeared fully in 57.5 percent of all cases and were reduced in 41 percent of those treated. Only among 1.5 percent did the complaint remain unchanged. The findings of the study allowed the confirmation of a clearly positive effect on disorders of the liver-gall bladder system. “In addition, subjective tolerability, measured by the exceptionally low number of wide effects, can be deemed very good,” a summary of the study results states. The conclusion: “Positive effect oft he treatment with Legapas among on average 98.5 percent of all cases.”

41. The first placebo double-blind study
To promote the scientific research into healing plants also on a European level, the national associations for phytotherapy founded the European Scientific Cooperative on Phytotherapy (ESCOP) in 1989. The European umbrella organisation draws up plant monographs to standardise the approval of plant-based pharmaceuticals in the European states. The German member of ESCOP is the Association for Phytotherapy based in Cologne, of which Pascoe is a corporate member.
Independently of this, the company Pascoe systematically drove the research into the effectiveness and tolerability of its own pharmaceuticals forwards. In 1991, Pascoe presented the results of the first double-blind, placebo-controlled study on Neurapas. Neither the patients nor the therapists knew who was receiving the placebo or Neurapas. The plant-based remedy produced for more than twenty years in the same composition was prescribed especially for depressive moods and nervous unrest and was considered to be easily tolerated. A significant component of the remedy is amber. In the study, the effectiveness of Neuropas was tested in an eight-week treatment of depressive disorders, as double-blind with a placebo, on 30 patients per group. A very good improvement of symptoms was noted as opposed to the placebo.
Pascoe also had Pascotox Forte-Injektopas studied, a prophylactic for increasing endogenous defences against bacterial and viral infections. The main component of this plant-based pharmaceutical is a liquid extract from the fresh Echinacea-pallida root. A clinic specialised in holistic immunobiological therapies carried out the study among 1000 in-patient admissions. The hospital primarily treated terminally ill cancer patients and used Pascotox as part of the fever and autohaemotherapy. The patients themselves recorded their body temperature, pulse, weight, nausea, vomiting and bleeding. Especially after autohaemotherapy, the wellbeing of patients improved. Unwanted side effects were not identified and the stimulation therapy was evaluated as having excellent tolerability. By 1995, Pascoe presented 85 retrospective studies, including on healing plants such as milk thistle, Echinacea, amber, gingko, garlic and valerian. The question was always: how and why the studied pharmaceuticals achieved their beneficial effect.
«… that today Pascoe has a unique competence Germany-wide when it comes to vitamin C.» Jürgen F. Pascoe

42. With Pascorbin on the way into the 21st century
Preventing diseases and strengthening the immune system became increasingly important in the 1990s. People were paying greater attention to their health; just the right time for Pascoe to launch the high-dose vitamin C product Pascorbin on the market. Several studies by the important American chemist Linus C. Paulig had shown that high-dose vitamin C not only supported metabolic processes and reduced the effects of toxic substances but also delayed the development of certain diseases and improved the general wellbeing of patients. “Pauling’s research results convinced us too,” Jürgen F. Pascoe explains. “We developed our own knowledge of ascorbic acid to such an extent that today Pascoe has unique competence Germany-wide when it comes to vitamin C.” A vitamin C deficiency is more common than one might think – especially among those who are chronically ill. The high-dose infusions are therefore justified in modern medicine. As an injection, high-dose vitamin C is offered up until today only by Pascoe.
A further milestone in corporate history was moving to the three-storey administrative new build in 1996. Pascoe was also embarking on new avenues in public relations. “Computer technology is my hobby,” Jürgen F. Pascoe recounts. “And therefore our company was also among the first three pharmaceutical manufacturers in Germany offering their customers a website.” For a wider public, Pascoe publishes a newsletter and the magazine Naturmedizin im Spektrum. Illustrated with graphs and tables, it offers interesting facts about medical history, alongside interviews, research and practice reports. In light of continuous investments into the modernisation of the company, product quality, research and development, Pascoe was well set up on the threshold to the new millennium. The corporate philosophy had paid off in every respect: to offer quality that other manufacturers could not provide, the use of high-quality raw materials, in-house production and the checking of the standardised substance properties. The central concern for Inge Pascoe and her two children Dr. Birgit Wilrich and Jürgen F. Pascoe was always: to maintain the big successes with biological-natural remedies whilst still being open to the progress of pharmacological research, if these can be brought together in a therapy without risk. This attitude significantly contributed to the success of the 1990s.

43. Nobel prize winner Linus C. Pauling (1901-1994) and vitamin C
Scurvy, a deficiency syndrome that starts with bleeding gums and can end with death through heart failure, was a widely feared disease among seafarers. It has been known since the 16th century that lemon juice helps against this. The healing substance that the human body cannot produce itself was first referred to as vitamin C in 1920. The vitamin suddenly became widely known in the 1970s through the book “Vitamin C and Colds” by the two-time Nobel Prize winner Linus Carl Pauling. It is about the positive effect of high-dose vitamin C on the human organism. Pauling was convinced that it could prevent many illnesses, improve general health and even prolong life.
The grandson of German immigrants and the son of a chemist researched for a long time the connection between illnesses and a vitamin and mineral deficiency. In 1954 he had received the Nobel Prize for Chemistry for his research into the molecular structure of proteins, in 1963 the Nobel Peace Prize for his efforts to end nuclear weapons testing. Apart from Marie Curie, Linus C. Pauling is the only person to have been awarded Nobel Prizes in two different categories. In 1973, together with two other scientists, he founded the Institute for Orthomolecular Medicine, the present-day Linus Pauling institute, at Oregon State University. It is dedicated to the research of vitamin and nutrient therapies for the prophylaxis and treatment of diseases.